On the image: Carl Diel, PhD, Senior Scientist, Manager NMR Services
Protein & Peptide BioNMR Discovery Services
SARomics Biostructures offers a broad range of custom peptide and protein NMR spectroscopy (BioNMR) analysis services for protein structure determination and fragment screening. Among our services are the following:
Our technology center answers some of the questions about the practical aspects of BioNMR experiments. You are also welcome to contact us directly to discuss the project.
- Peptide NMR & protein NMR assignment, secondary structure assessment
- Peptide NMR & protein NMR structure determination
- Protein-ligand & protein-protein complex structure determination
- Fragment screening using 1D or 2D NMR spectroscopy
- Epitope mapping and dissociation constant (Kd) determinations for compounds
- Biosimilars higher order structure (HOS) comparability analysis, formulation characterization and optimization
- Expression and purification of 2H, 15N, and 13C-labelled protein in E.coli for NMR spectroscopy studies and structure determination
Our technology center answers some of the questions about the practical aspects of BioNMR experiments. You are also welcome to contact us directly to discuss the project.

Custom high-resolution NMR structure determination and analysis
Peptide or protein BioNMR is a powerful alternative to X-ray crystallography for determining and analyzing protein three-dimensional structures. Especially for peptides, where crystallization often is essentially impossible. In contrast to X-ray crystallography, analysis by NMR spectroscopy only requires the peptide or the protein to be stable in solution to determine its structure. Generally, only proteins up to 30 kDa in size can be determined using NMR. Our custom NMR structure determination services are typically done using iterative restrained molecular dynamic simulations with structural restraints generated from 2D and 3D NMR spectroscopy experiments.

Peptide and protein assignment and secondary structure analysis using NMR
The first step in peptide or protein NMR spectroscopy structure analysis involves assigning the detected peaks in the NMR spectrum to individual atoms. The assignment can be used for various purposes such as primary structure determination and secondary structure assessment for peptides, assignment of 2D 15N NMR spectra for epitope mapping, fragment screening, and Kd determinations. The assignment also provides information on whether a structure determination of a peptide or a protein is possible.

Custom protein-ligand/protein-protein complex NMR structure determination
Structure analysis of protein complexes with BioNMR can be performed if the assignment of the protein is available. Generally, de novo structure determination of a complex is possible even if the structure of the components is unknown. Otherwise, the complex can be analyzed using available assignments and structures (X-ray or NMR) in combination with filtered NOESY experiments or transfer NOE experiments. The protein needs to be labeled with 15N and 13C. In contrast to X-ray crystallography, even complexes where the binders have affinities in the µM-mM range can be analyzed, which can be particularly suitable for peptide-protein and protein-protein complexes.

NMR fragment and ligand screening
We regularly run custom NMR fragment and ligand screening and validation of drug-like compounds in combination with fragment screens using our proprietary weak-affinity chromatography (WAC™) platform or as a stand-alone screening method. We can run either 1D ligand-detection experiments or 2D spectra, depending on the target protein.
For 1D ligand detection, we can run either 1H or 19F experiments. Competition experiments with a known binder allow the identification of potential binding sites. For 2D experiments, generally, the protein needs to be labeled with 15N (for large protein 2H-labelling as well). However, recent advances in NMR technology make it possible to run NMR fragment screening on non-isotope labeled samples by looking at the methyl region of the 13C NMR spectrum. If the assignment is available, the binding epitope of various fragments can be readily identified at atomic resolution.
For 1D ligand detection, we can run either 1H or 19F experiments. Competition experiments with a known binder allow the identification of potential binding sites. For 2D experiments, generally, the protein needs to be labeled with 15N (for large protein 2H-labelling as well). However, recent advances in NMR technology make it possible to run NMR fragment screening on non-isotope labeled samples by looking at the methyl region of the 13C NMR spectrum. If the assignment is available, the binding epitope of various fragments can be readily identified at atomic resolution.

Epitope mapping and dissociation constant (Kd) determinations for compounds
The binding epitope of a compound can be readily analyzed with BioNMR using 1D or 2D experiments, where the choice of the experiment depends on the availability of isotope-labeled protein and peak assignments. For 1D experiments, ligand-detection NMR experiments in combination with competition experiments using a known binder can identify the binding site of a compound. For 2D experiments, the binding site of a compound can be identified at an atomic resolution if an assignment is available. However, even without assignment, it is possible to identify the binding site if a known binder is available. In a similar way, the dissociation constant (Kd) can be determined using titration experiments detected by 1D or 2D NMR. For 2D experiments, no assignment is needed for Kd determinations. However, it is beneficial if atomic resolution is required.

Higher order structure (HOS) comparability analysis of biosimilars, comparability studies for formulation optimizations
NMR spectroscopy can be used to analyze higher order structure (HOS) of biosimilars and to compare a biosimilar's structure to its originator antibody. For this purpose, 2D 13C NMR spectra of the methyl region of the biomolecule are acquired, where the biosimilar and originator spectra can be compared. No labeling is required, and molecules as large as full antibodies can be analyzed using this approach. In addition, the biomolecules can be studied in the formulation buffer for formulation optimization and batch comparison. Depending on the biomolecule, around 1-2 mg of protein can be sufficient for HOS analysis.
Please see also our recent blog post on why NMR spectroscopy is the best method for the comparability study of biosimilars.
Please see also our recent blog post on why NMR spectroscopy is the best method for the comparability study of biosimilars.
Expression and purification of 2H, 15N and/or 13C-labelled protein in E.coli for NMR studies
For BioNMR peptide and protein structure determination, the protein needs to be labeled with 15N and/or 13C to assign the 2D NMR spectra at atomic resolution. This is readily done by expression in E. coli using in-house protocols, where especially 15N-isotope labeling is no more expensive than a standard E. coli expression. In addition, 15N-isotope labeling is usually sufficient for running 2D NMR fragment screening and can also be used to verify the folding state of protein constructs. Depending on the project, additional isotope labeling might be required, especially for larger proteins that usually yield better NMR spectra when they are 2H labeled.
Recent publications
Petruk G, Puthia P, Samsudin F, Petrlova J, Olm F, Mittendorfer M, Hyllén S, Edström D, Strömdahl A-C, Diehl C, Ekström S, Walse B, Kjellström S, Bond PJ, Lindstedt S & Schmidtchen A (2023).
Targeting Toll-like receptor-driven systemic inflammation by engineering an innate structural fold into drugs.
Nat Commun, 14, 6097. https://doi.org/10.1038/s41467-023-41702-y
PDB entries:
8BWW - Targeting Toll-like receptor-driven systemic inflammation by engineering an innate structural fold into drugs
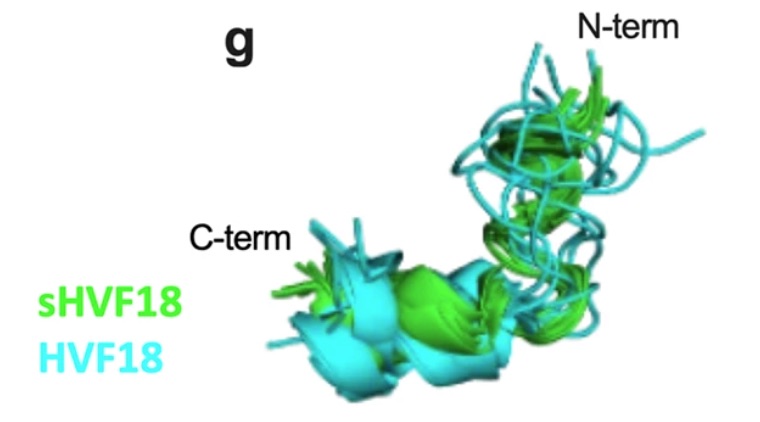
Thrombin-derived C-terminal peptides (TCPs) are endogenous anti-infective immunomodulators interfering with CD14-mediated TLR-dependent immune responses. Using a combination of structure- and in silico-based design, nuclear magnetic resonance (NMR) spectroscopy, biophysics, mass spectrometry, cellular, and in vivo studies, the paper presents the structure, CD14 interactions, protease stability, transcriptome profiling, and therapeutic efficacy of sHVF18.
For a full list please visit our publications page.
Targeting Toll-like receptor-driven systemic inflammation by engineering an innate structural fold into drugs.
Nat Commun, 14, 6097. https://doi.org/10.1038/s41467-023-41702-y
PDB entries:
8BWW - Targeting Toll-like receptor-driven systemic inflammation by engineering an innate structural fold into drugs
Thrombin-derived C-terminal peptides (TCPs) are endogenous anti-infective immunomodulators interfering with CD14-mediated TLR-dependent immune responses. Using a combination of structure- and in silico-based design, nuclear magnetic resonance (NMR) spectroscopy, biophysics, mass spectrometry, cellular, and in vivo studies, the paper presents the structure, CD14 interactions, protease stability, transcriptome profiling, and therapeutic efficacy of sHVF18.
For a full list please visit our publications page.