Antibody Structure & Comparability Analysis Services
SARomics Biostructures' team has extensive experience in X-ray and NMR spectroscopic analysis of monoclonal antibody and antibody-antigen complex structures. Our services also include structural characterization and comparability studies of biosimilars' higher-order structure (HOS) using 13C-natural abundance 2D-NMR spectroscopy. All our studies include biophysical characterization of the state of the monoclonal antibodies and biosimilars in solution.
More detailed, our monoclonal antibody structure services can help you with the following:
Please see our blog series on monoclonal antibodies and biosimilars for additional information. Our X-ray crystallography and NMR spectroscopy services platform is equipped to handle projects of all types, including challenging projects. To discuss your project, please do not hesitate to contact us.
For an overview of our services, please download the white paper below.
More detailed, our monoclonal antibody structure services can help you with the following:
- Epitope definition to file stronger IP
- Understanding the mode of action
- Structure-based antibody engineering: affinity maturation, humanization, antibody-drug conjugates (ADC), etc.
- Structural characterization of protein drugs for regulatory purposes
Please see our blog series on monoclonal antibodies and biosimilars for additional information. Our X-ray crystallography and NMR spectroscopy services platform is equipped to handle projects of all types, including challenging projects. To discuss your project, please do not hesitate to contact us.
For an overview of our services, please download the white paper below.
Case studies
The PDF file below provides a short company introduction and shows several case studies involving antibody-antigen complex structures. They were published recently in leading journals such as PNAS, Nat Comm, Cell Reports, iScience, Cancer Ther, Blood Ad, and Structure. The determined structures include:
Our publications page provides additional examples.
- Bispecific anti-Met/EpCAM mAb MM-131 in complex with its antigens (Merrimack Pharmaceuticals).
- ALPN-202 (An engineered CD80 variant fusion therapeutic) in complex with PD-L1 (Alpine Immune Sciences).
- SRF388 Fab in complex IL-27 (Surface Oncology Inc.),
- ActRIIB-Alk4-Fc in complex with activin A and anti-ActRIIB Fab (Acceleron Pharma).
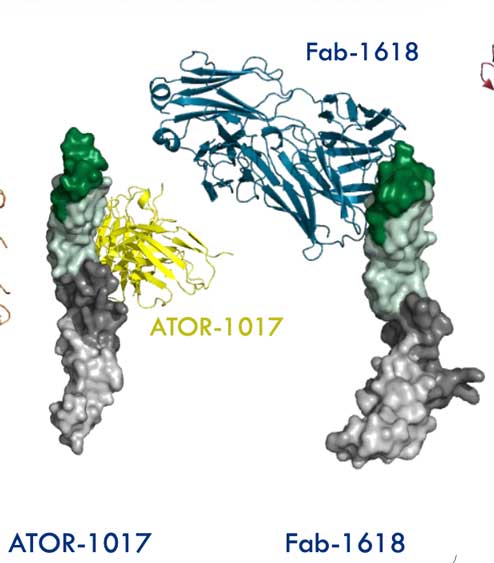
- ALG.APV-527 (Fab1618) in complex with 4-1BB (CD137) (Alligator Bioscience AB).
- Glenzocimab Fab in complex with platelet glycoprotein VI (Acticor Biotech),
- DutaFab (Roche) in complex with its antigens PDGF and VEGFA.
- Dusquetide in complex with p62 (SQSTM1) ZZ domain (Soligenix, Inc.).
Our publications page provides additional examples.

On the image: Martin Welin, PhD, Senior Scientist, Team Leader, Protein Crystallography.
Comparability analysis of Biosimilars using NMR spectroscopy
Assessing the comparability of a therapeutic monoclonal antibody's higher-order structures (HOS) and its biosimilar is critical to developing new products that adhere to patient safety principles. The NMR-based approach offered by SARomics Biostructures is currently the most efficient HOS comparability assessment approach at atomic resolution. Please see our latest blog post on which experimental method is best for assessing the structural comparability of a biosimilar and its reference antibody.
Our analyses are based on advances in NMR spectroscopy that have made it possible to acquire a unique fingerprint representation of the 3D conformation of large, complex molecules like biologics. For biosimilar comparability assessment, 2D 13C NMR spectra of the methyl region of the biomolecules are acquired, and the biosimilar and originator spectra are compared. The method is based on natural abundance and does not require additional expensive labeling. Molecules as large as whole antibodies can be analyzed using this approach. By directly matching the NMR fingerprint of a given protein to its high-resolution 3D structure, determined, e.g., by X-ray crystallography, or to a fingerprint of another protein batch of the therapeutic monoclonal antibody or its biosimilar, we can rapidly assess and analyze comparability and show that the molecules, for example, a biosimilar and its originator, or different batches or alternative preparations of the same biologic, have identical HOS. In addition, the biomolecules can be studied in the formulation buffer for formulation optimization and batch comparison. Depending on the biomolecule, around 1-2 mg of protein can be sufficient for HOS analysis.
Our method can substantially accelerate the characterization of monoclonal antibody structures, helping you save both time and money. Additional details on the NMR experiments can be found on our NMR services page.
You may also download the NMR spectroscopy HOS services white paper and a pdf of a presentation "Higher order structure (HOS) assessment, comparability and characterization using NMR spectroscopy" with case studies.
Our analyses are based on advances in NMR spectroscopy that have made it possible to acquire a unique fingerprint representation of the 3D conformation of large, complex molecules like biologics. For biosimilar comparability assessment, 2D 13C NMR spectra of the methyl region of the biomolecules are acquired, and the biosimilar and originator spectra are compared. The method is based on natural abundance and does not require additional expensive labeling. Molecules as large as whole antibodies can be analyzed using this approach. By directly matching the NMR fingerprint of a given protein to its high-resolution 3D structure, determined, e.g., by X-ray crystallography, or to a fingerprint of another protein batch of the therapeutic monoclonal antibody or its biosimilar, we can rapidly assess and analyze comparability and show that the molecules, for example, a biosimilar and its originator, or different batches or alternative preparations of the same biologic, have identical HOS. In addition, the biomolecules can be studied in the formulation buffer for formulation optimization and batch comparison. Depending on the biomolecule, around 1-2 mg of protein can be sufficient for HOS analysis.
Our method can substantially accelerate the characterization of monoclonal antibody structures, helping you save both time and money. Additional details on the NMR experiments can be found on our NMR services page.
You may also download the NMR spectroscopy HOS services white paper and a pdf of a presentation "Higher order structure (HOS) assessment, comparability and characterization using NMR spectroscopy" with case studies.
Recent publications
Karin Enell Smith, Sara Fritzell, Anneli Nilsson, Karin Barchan, Anna Rosén, Lena Schultz, Laura Varas, Anna Säll, Nadia Rose, Maria Håkansson, Laura von Schantz & Peter Ellmark (2023).
ATOR-1017 (evunzekibart), an Fc-gamma receptor conditional 4-1BB agonist designed for optimal safety and efficacy, activates exhausted T cells in combination with anti-PD-1.
Cancer Immunology, Immunotherapy, published online ahead of print.
https://doi.org/10.1007/s00262-023-03548-7
ATOR-1017 (evunzekibart), an Fc-gamma receptor conditional 4-1BB agonist designed for optimal safety and efficacy, activates exhausted T cells in combination with anti-PD-1.
Cancer Immunology, Immunotherapy, published online ahead of print.
https://doi.org/10.1007/s00262-023-03548-7
Our collaborator Alligator Bioscience demonstrates that the unique epitope of ATOR-1017 combined with an IgG4 Fc can potentially induce 4-1BB-mediated T cell and NK cell activation. The crystal structure of the complex between human 4-1BB and ATOR-1017 as a scFv was determined by SARomics.
You may also view the poster presented by the SARomics' team at the Festival of Biologics meeting in Basel, Switzerland:
For the complete publication list, please visit our publications page.