This is the third article in our series about therapeutic monoclonal antibodies. This time, we focus on structural characterization and comparability analysis of biosimilars.
As noted in the previous article, biologics are much more complex molecules compared to small-molecule drugs. They are produced in living cells and may undergo uncontrolled post-translational modifications (PTM) and glycosylation, which may affect the three-dimensional structure of the drug and modify its biological potency. Other factors, like changes in pH, ionic strength, buffers, or the presence of various additives in the formulation solution, may also affect the structure. All these factors must be considered in developing biosimilars, the follow-on versions of a therapeutic monoclonal antibody (once patent protection of the original drug has expired). The costs of biosimilars, like those of generic drugs, are expected to be lower, making them more affordable in clinics.
The general assessment of biosimilars' comparability to the reference drug must involve physicochemical and functional characterization, the study of animal toxicity, human pharmacokinetics/pharmacodynamics, immunogenicity, clinical safety, and effectiveness. When considering physicochemical quality attributes, the regulatory requirements list three essential factors - PTM, higher order structure (HOS, i.e., secondary, tertiary, and quaternary structure) comparability, and protein aggregation. While the techniques for detecting, characterizing, and quantifying PTMs and aggregates are well known and applied efficiently to antibody characterization, assessing possible structural differences between the biosimilar and its originator that may arise due to PTM, glycosylation, or other factors is still challenging. X-ray crystallography and NMR spectroscopy are the primary protein structure-determination techniques, with cryo-EM rapidly catching up. However, opening a well-cited review on the subject published in Nat Rev Drug Disc, 2012, we can read that "…the application of these technologies (crystallography and NMR) for higher-order structural comparability studies presents major problems". Eight years later, in a 2020 paper published in Generic & Biosimilars Journal, we can still read that "Classical X-ray diffraction can only be used with solid-state materials" and it is "not suitable for pharmaceutical products which are commonly produced in solution"! What about the 5500+ antibody X-ray structures in the PDB or SabDab database? Let us also imagine how much we would know about antibodies without having access to their X-ray structures or where small-molecule drug discovery would be today without NMR spectroscopy and X-ray crystallography! Not surprisingly, NMR is also rejected as a method for comparability studies despite the developments that showed that NMR fingerprinting techniques based on natural abundance can be used time-efficiently for monoclonal antibody HOS characterization (Poppe et al., 2013; Poppe et al., 2015). In addition, the feasibility of NMR-based mapping of monoclonal antibody structure using a 2D 13C NMR methyl fingerprint method was also shown by Arbogast et al., 2014. The authors showed that a single 2D-NMR experiment, rapidly acquired in as short as approximately 30 min, can yield a spectral map that offers an atomic-level fingerprint of a protein therapeutic's primary, secondary, tertiary, and quaternary structure. The fingerprints of the reference antibody and its biosimilar are subsequently compared to verify the level of similarity between the structures.
What are the standard techniques currently suggested for use in HOS comparability analysis? The authors of the cited 2020 review in Generic & Biosimilars Journal refer to the 2011 'Guidelines for the practical stability studies of anticancer drugs: A European consensus conference' in which it is recommended to "use at the minimum one method to assess the integrity of the secondary structure and another assess tertiary structure". And "specifically, second derivative FTIR (Fourier-transform infrared) and UV are recommended, together with a global evaluation that should be performed through a thermodynamic stability study". While FTIR is suggested for assessing the secondary structure, UV spectroscopy (tryptophan fluorescence) is suggested for assessing the integrity of the tertiary structure.
Without going into the details of these methods and what they measure, and the recent history of the development of NMR methods, we jump to a couple of papers, the first one from 2020 with the title "A Comparison Between Emerging and Current Biophysical Methods for the Assessment of Higher-Order Structure of Biopharmaceuticals" published in J Pharm Sc, 2020, by Mats Wikström and colleagues at Amgen, California. The authors used a set of samples of monoclonal antibodies belonging to the subclasses IgG1 and IgG2 mixed at different proportions to study the capabilities of the different methods to detect the presence of the two IgGs. The authors note that the amino acid sequence identity between the two samples was 95%, meaning that the mixture containing, e.g., 90% of IgG1 and 10% of IgG2 corresponds to a minor difference in the overall structural properties. This suggests that if the differences are still detected in this sample, the setup can be considered appropriate for evaluating the differentiating ability of the experimental methods.
The authors compared the commonly recommended methods, FTIR, circular dichroism (CD), UV fluorescence spectroscopy, and differential scanning calorimetry (DSC), to 1D 1H Profile NMR. They showed that NMR can distinguish between most sample combinations (93%), DSC can differentiate 61%, and CD spectroscopy can differentiate 52% of the combinations. In contrast, no significant distinction between any samples using FTIR or intrinsic fluorescence could be made. The results speak for themselves; the ability of NMR spectroscopy to distinguish between samples was proved far superior to all other methods, especially the spectroscopic methods, which are essentially useless in this application. Another study (Brinson et al., 2019) involved 26 industrial, government, and academic laboratories. It aimed to establish benchmarks for adopting the 2D NMR technique performed under controlled sample conditions for accurate measurements and comparison of biopharmaceutical HOS. The experimental results showed that the method is reliable and can be harmonized for routine measurements with highly accurate assessments of the HOS of monoclonal antibody therapeutics.
How is structural comparability studied at SARomics?
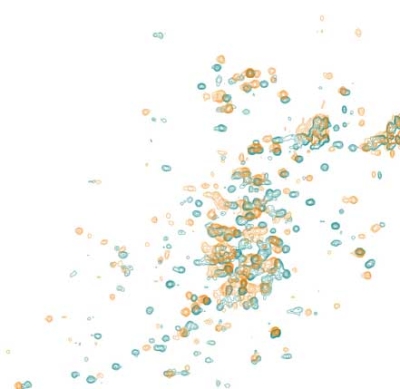
At SARomics Biostructures, we use the 2D 13C NMR method described above. The fingerprint spectra of the methyl groups of the originator biomolecule and its biosimilar are registered and compared, and any differences between the two are directly revealed. A significant advantage is that no extra labeling is required for these experiments, meaning no additional material must be produced. Molecules as large as whole antibodies can be analyzed using this approach. In addition, the technique can be used for formulation optimization and batch comparison. In such cases, the biomolecules can be studied in the actual formulation buffer. Depending on the biomolecule, around 1-2 mg of protein protein can be sufficient for HOS analysis.
Please visit our antibody structure services page or download a PDF with a short company presentation and antibody-related case studies.